NORD supports robust, well-funded newborn screening programs in every state.
Newborn screening originated in the 1960s when Dr. Robert Guthrie developed a blood test for phenylketonuria (PKU), a serious and rare metabolic disorder that causes brain damage if not detected and treated early in life. Children with PKU appear healthy at birth, but they are born without an enzyme necessary to break down certain proteins. As a result, an amino acid called phenylalanine builds up in the body, causing permanent damage. Before Dr. Guthrie’s blood test, children with PKU were not diagnosed until after they had developed irreversible brain damage. The blood test allowed health care providers to detect PKU shortly after birth, enabling earlier treatment and avoiding serious health complications caused by the condition.1
Today, nearly 4 million newborns are screened annually in the U.S. for certain rare conditions that, like PKU, can cause permanent disability or death without early detection and treatment.2 All 50 states currently mandate screening for at least 32 serious rare conditions. The specific conditions screened are determined by each state, with most programs currently screening for a majority of the 38 conditions included on the federal Recommended Uniform Screening Panel (RUSP). If a screen comes back positive, the health department notifies the newborn’s health care provider. The newborn’s health care provider is then informed and the appropriate follow-up measures are set into motion.
This section of the State Report Card evaluates each state on whether they have adopted policies that are key to operating a successful and impactful newborn screening program.
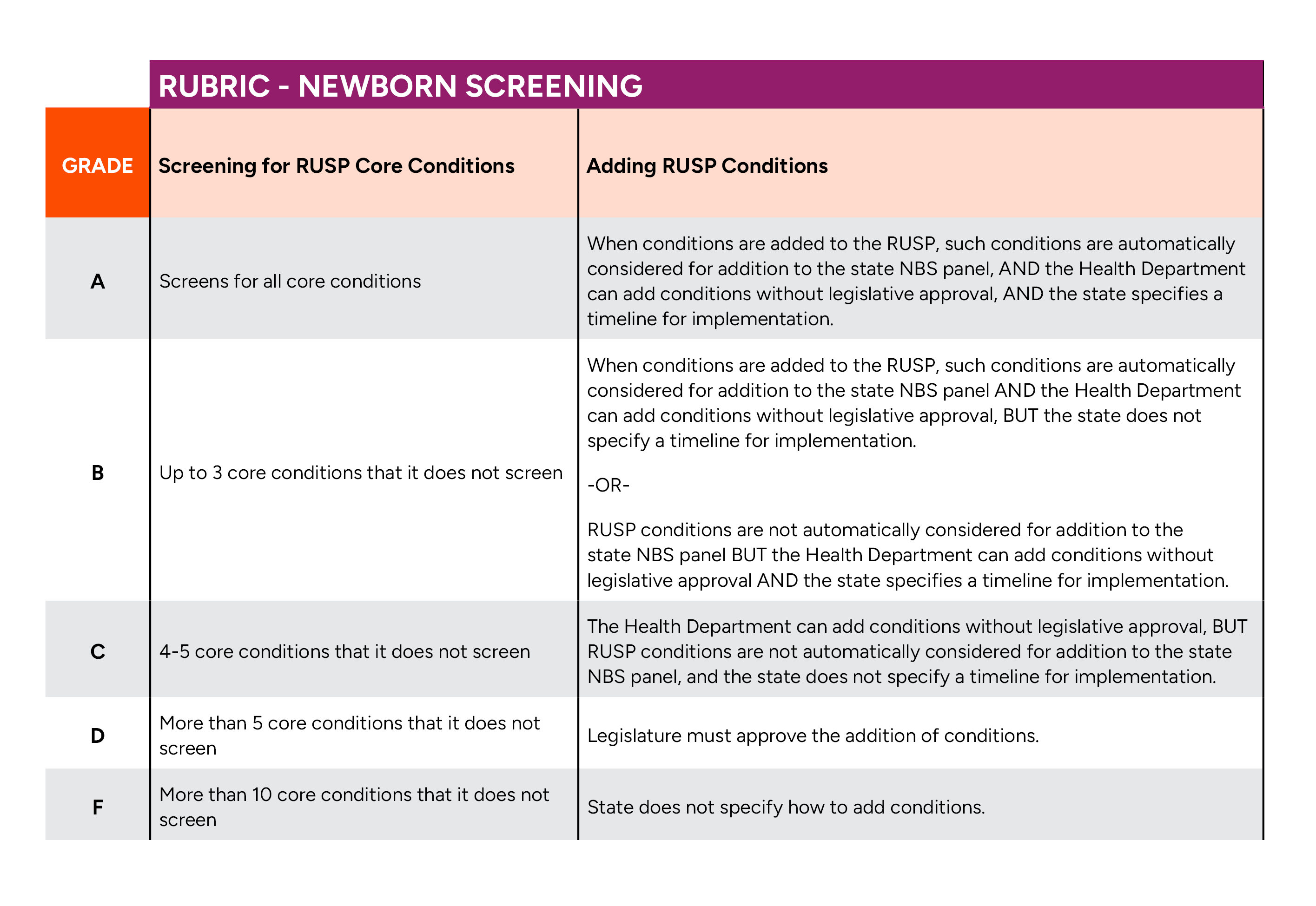
Screening for RUSP conditions
Newborn screening programs are regulated and administered at the state level, which allows each program to be customized to fit the state’s specific needs. The U.S. Department of Health and Human Services (HHS) also plays a role by administering the Recommended Uniform Screening Panel (RUSP), a set of recommended conditions for which HHS has determined the condition to be appropriate for screening. To date, the RUSP consists of 38 rare conditions, as well as screening for congenital heart disease and hearing loss.3 Many states also screen for additional serious conditions detectable through screening at birth known as “secondary conditions.” Each state determines which RUSP and secondary conditions are included on the state’s newborn screening panel.
NORD believes that states should screen for all RUSP conditions and assesses states based on the number of RUSP conditions included on the state’s newborn screening panel. The more RUSP conditions screened by a state, the higher the grade the state receives.
Adding RUSP core conditions
As new screening methods, treatments, and cures become available for serious rare disorders, additional conditions become good candidates for newborn screening. The number of conditions on the RUSP grows each year as conditions are added following a rigorous nomination and review process. The most recent condition added to the RUSP was Krabbe disease in 2024.4
NORD believes that it is important for states to have a plan in place to efficiently add new conditions to their own state screening panel once they are added to the RUSP. For most states, the addition of a condition to the newborn screening panel depends on several factors, such as budget conditions and the prevalence of the condition. Additionally, the process for adding conditions can be time intensive. In many states, there is usually a pilot period where the state rolls out adding the new condition to the testing panel in phases to ensure they have the proper processes and tools in place to handle the additional workload. The pilot can take months or years to complete. States with efficient and effective processes to add conditions to their state panel once they have been included on the RUSP received a higher grade.
1. About newborn screening. Newborn Screening. December 2023. Accessed July 16, 2024. https://newbornscreening.hrsa.gov/about-newborn-screening.
2. Gaviglio A, McKasson S, Singh S, Ojodu J. Infants with Congenital Diseases Identified through Newborn Screening-United States, 2018-2020. Int J Neonatal Screen. 2023;9(2):23. Published 2023 Apr 13. doi:10.3390/ijns9020023
3. Federal Advisory Committee. (August 2022). Recommended Uniform Screening Panel. Health Resources & Services Administration. https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp
4. Ibid.
Click to enlarge.

Every day matters for patients, researchers, and families who rely on NORD's trusted information.
We can’t keep these resources free and growing without your help.
Please donate today — and power the future of rare disease progress.


Whether you’re a patient, researcher, student, or advocate — we’re here for you.
If you’ve found NORD’s resources helpful, please consider donating.
Your support keeps this information free, updated, and available for everyone who needs it.

